Debye Length
DEFINITION
Debye length is an important characteristic of the interfacial electric double layers. It serves as a measure of the double layer thickness. Consequently, it depends mostly on ionic strength. Increasing ionic strength reduces Debye length, makes double layer thinner. Such reduction of the Debye length leads to weaker electrostatic interactions. Therefore, this could cause stability loss by dispersions and emulsions.
Transition from the fresh to sea water at the rivers deltas is striking example illustrating importance of this parameter. This change causes aggregation of particles when they float in the river waters and consequent formation of the deposits at the rivers deltas.
METHOD of CALCULATION
There is a simple method for estimating Debye length using conductivity measurement suggested by Dukhin and Goetz. It is described in International Standard ISO 13099, Parts 1: 2012 “Colloidal systems – Methods for Zeta potential determination”
Following expression presents formula for such calculations, where conductivity is K:
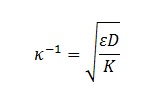
where ε is dielectric permittivity, and D is diffusion coefficient
The main uncertainty in this equation comes from the effective diffusion coefficient D because it is usually unknown. However, this parameter varies over limited range. For instance, the diffusion coefficients of most ions in aqueous solutions are similar. Their values that at room temperature are in the range of 0.6×10-9 to 2×10-9 m2/s. The square root of this variation would yield uncertainty in scale of only tens precents. With regard to non-aqueous systems, recent studies indicate that ions in such liquids are not much larger either.
MEASUREMENT DEVICES
Obviously, application of this method for ANY liquid requires capability of measuring conductivity within complete liquids conductivity range from 10-11 S/m up to 10 S/m. We have several instruments that cover this very wide dynamic range:
- DT-700 for low conductivity range below 10-4 S/m;
- DT-330, DT-350, DT-900 for high conductivity range above 10-4 S/m. Alternatively one can use Options OP004 and OP0041 for the Model DT-1202.
