A NEW WAY TO CHARACTERIZE STABILITY
AND PERFORMANCE OF COSMETIC EMULSIONS
AND SUSPENSIONS
David Fairhurst, Ph.D., Andrei Dukhin, Ph.D., and Kenneth Klein, M.S.
ABSTRACT
Cosmetic emulsions, (as all macro-emulsions), are inherently unstable systems, (from a thermodynamic viewpoint). Achieving a small average particle size along with a narrow particle size distribution will typically result in a more stable and elegant product. Two reliable and well-established parameters that are intimately related to the physical stability of an emulsion are, particle (droplet) size and zeta potential (surface charge). Unfortunately, current instrumentation is severely limited in its application to cosmetic emulsions because of the requirement to dilute the system under investigation. Cosmetic emulsions are complex, multi-component systems that, invariably, cannot be diluted without effecting their particle size and hence stability. Additionally, product performance characteristics such as spreadability and efficacy, (such as SPF), may be enhanced by reducing the particle size. We report a new technique based on the use of acoustic spectroscopy in combination with electro-acoustics to measure particle size and zeta potential in both O/W and W/O emulsions without the need for dilution. The advantages and limitations of the method will be briefly reviewed and data presented illustrating its relevance to a variety of cosmetic emulsions from sunscreens to moisturizers
*************
Introduction:
The design of a cosmetic emulsion is quitecomplex and often requires several iterations to produce a stable, efficacious, safe, cost effective and elegant product. While product specifications are established to insure product consistency during manufacture, all too often they are not sufficient to ensure that a truly high quality product has been manufactured. What is needed is an objective test(s) that will be easy to run, and predictive – a test that measures fundamentalcharacteristics in nature (1,2). They must neither be a function of the instrument used not of the operator.
Two reliable and well-established parameters are, respectively, particle (droplet) size and zeta
potential (surface charge) (3,4). The techniques devised to determine them are extremely diverse. Each
method has its attractions (5,6) but it is important to select the one that meets the actual requirements of
the application in question; versatile as many current instruments may be, the one that does everything ____________________
Dr. David Fairhurst
is Executive Vice President of Particle Sciences Inc., Bethlehem, Pennsylvania. Dr. Andrei Dukhin is President of Dispersion Technology Inc., Mount Kisco, New York. Kenneth Kleinis President of Cosmetech Inc., Fairfield, New Jersey.
has yet to be invented. Unfortunately, the vast majority of current instrumentation utilizes light
scattering. This severely limits the application to cosmetic emulsions because of the requirement to dilute the system under investigation.
Cosmetic emulsions are complex, multicomponent systems that, invariably, cannot be diluted without consequence. The (dilution) process not only destroys any structure characteristics but also can induce instability leading to creaming and, often, breaking/coalescence. Hence the oft-found difficulty of correlating particle size (PS) and zeta potential (ZP) measurements with flow properties, freeze-thaw and shelf storage behavior. PS, alignment of emulsifier at the interface and thus ZP may be influenced by many factors:
1. Emulsifier choice
2. Emulsifier placement
3. Emulsifier concentration
4. Manufacturing temperature
5. Manufacturing techniques
a. Add internal phase to external phase
b. Add external phase to internal phase and undergo a phase inversion
c. One “pot” technique
d. Shock emulsification
e. High shear
6. Phase volume ratios
Additionally, many instruments are only suitable for studying O/W emulsions, or may have difficulty evaluating emulsions that contain significant concentrations of dispersed particulates, as may be found in sunscreen formulations containing Zinc Oxide/Titanium Dioxide.
It has been long known that ultrasound based techniques open an opportunity to eliminate dilution (7-10). However, instrumentation hardware was too complex and suffered from weaknesses in practical application of the technique to systems of high internal phase concentration. In addition, the theoretical basis of the deconvolution of the raw data was not capable of dealing with particle-particle interaction – an essential feature of concentrated dispersions. Recently, all these problems have been addressed and a commercial instrument developed that convincingly demonstrates the advantages of a technique based on a combination of acoustic spectroscopy and electro-acoustics (11,12). This offers a new opportunity for characterizing these complicated cosmetic systems; it gives an advantage of being able to study turbid emulsions where other techniques such as light scattering simply will not work and dilution of such systems can alter their physical properties. It is also sensitive enough for characterizing polydispersity. A major advantage of a technique based on sound is that a sample under investigation need not be stationary, a necessary requirement of light scattering instrumentation. Hence, an acoustic instrument holds promise of being able to study emulsions that are flowing, in-line or on-line. The technique has been successfully applied to the study of simple (3 component), model emulsions (13,14). Unfortunately, model systems, while of academic interest to the researcher, are often of little practical use to the cosmetic formulator.
In this paper we report the results of a preliminary study of a variety of “real-world” cosmetic emulsions, both O/W and W/O, from sunscreens to moisturizers. The aim of the study was to determine the limitations of the acoustic method and to show proof-of-concept in the ability of the technique to help characterize the stability and performance of cosmetic emulsions without the need for dilution.
Experimental:
A series of emulsions were obtained from Cosmetech Laboratories Inc., Fairfield, New Jersey. They were formulated to be commercially viable cosmetic products. The details of the systems are given in Table 1. The emulsions varied widely in composition and character viz internal phase wt. fraction, viscosity and stability.
The instrument used was a DT-1200 Acoustic Spectrometer developed by Dispersion Technology, Inc., Mt Kisco, New York (15). This instrument has two separate sensors for measuring acoustic and electro-acoustic signals independently; details of the instrument and its operation are given elsewhere (13,14,). The raw data output is ultrasound attenuation frequency spectra within a frequency range from 1MHz to 100MHz that is known to be typical for heterogeneous systems. The attenuation spectra contain information about particle size distribution (PSD). The electroacoustic sensor raw data output is Colloid Vibration Current; this measured parameter yields information about ZP. Complete PSD and ZP measurements can be completed in minutes and can be made on flowing systems.
Results and Discussion:
Emulsion A
This was an anionic (glyceryl stearate + AMP stearate), basic O/W cosmetic emulsion. The measured zeta potential was found to be –20.0 mV (Table 2), a not unexpected value given the known facts of the emulsion and is quite typical of such systems (5). Results of the raw attenuation data are given in Figure 1. Curve A corresponds to an initial measurement of the emulsion at rest and curve B is the result after subjecting the emulsion to shear produced by peristaltic pumping for 30 hours. There is a marked change in attenuation indicating that the particle size distribution (PSD) has changed; the emulsion is not “stable” to the level of shear produced by the peristalsis. Figure 2 shows measurements made, at two different parts of the attenuation curve (frequencies 4MHz and 100MHz), as a function of time of shear. These time dependences are different and this reflects variation of different droplet sizes.
The low frequency attenuation (4MHz) drops rapidly and then levels (curve B). Attenuation at this frequency is mostly dependent only on the large droplets. The observed time dependence of an initial rapid drop in attenuation indicates that the shearing action has destroyed large size droplets.
The reduction in high frequency attenuation (100MHz) is much slower (curve A). Attenuation now depends on both small and large droplets. The longer time dependence of the high frequency attenuation is what would be expected if the small droplets were removed by, for example, coalescence. This is consistent with the removal of large droplets at the initial much shorter times.
Thus, we conclude that the original emulsion must be bimodal and the shearing action produces two effects: large droplets are broken down into smaller droplets and smaller droplets are coalescing. The overall result is depicted in Figure 3 showing the PSD for the emulsion before and after shear. Initially, the emulsion comprises primarily very small droplets (ca 70nm mean) with a fraction of droplets about 8 um. After shearing the PSD changes. There are fewer of the small droplets and considerably more larger droplets but the mean PS of the larger fraction is now around 4um. Thus, it is clear that, using acoustic measurements, dynamic changes in the state of an emulsion can be followed in real time
Emulsion B
This was a stable, nonionic (laureth-23) O/W waterproof sunscreen formulation. The initial measured ZP (Table 2) was +4.5 mV. This is surprising. Typically, nonionically stabilized suspensions have a ZP close to 0 mV but negative in sign (5). On examination of the formulation components for this emulsion, it was found to contain EDTA and TEA. Thus, the only explanation for the positive value of the ZP is that the either or both amines specifically adsorbed at the oil/water interface. This result warrants further investigation.
For the attenuation data we first measured the emulsion under quiescent conditions and then deliberately induced instability by diluting approximately 2.5 times with deionized water; the attenuation drops by a factor 10, reflecting coalescence and phase separation (Figure 4). We then added a surfactant, sorbitan laurate (SPAN 20), to try to effectively re-stabilize the emulsion, without any homogenization. The changes in attenuation spectra are shown in Figure 5. Although there was some improvement, the emulsion remained unstable. The ZP of the emulsion was re-measured as +3.0 mV confirming that the effect of simple addition of the surfactant was ineffective. It can be surmised that the sorbitan laurate required high energy (high shear or heat) in order to adsorb at the oil/water interface and effectively stabilize the emulsion. Again, the results demonstrate the ability of the acoustic method to characterize the behavior of an emulsion under different chemical conditions.
Emulsion C
This W/O emulsion was an unstable sunscreen formulation that was separated into two distinct phases. Pumping the emulsion into the DT-1220 allowed the two phases to mix. The raw attenuation spectra are shown in Figure 6.
The initial measurement (Curve A) results in a PS of 40um. 2% sorbitan laurate was added but this did little to improve the stability (Curve B); the PS remained around 40um and the “emulsion” was very thin and of low viscosity. Increasing the surfactant concentration to 5.5% (curve C) resulted in a dramatic change to a white emulsion of very high viscosity and a mean PS of only 2um.
Emulsions D and E
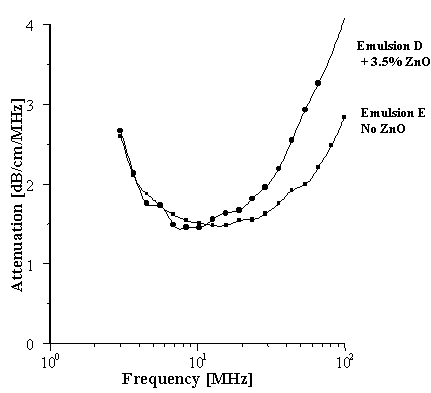
These emulsions are examples of nonionically stabilized O/W moisturizers, a major difference being that Emulsion E also contains 3.5% of a particulate sunscreen active, a microfine hydrophobically coated zinc oxide (ZnO). The raw attenuation spectra are compared in Figure 7 and are clearly distinguishable from one another. It is also apparent that the shapes of the two spectra are different. Work is currently in progress to determine if the PSD of the ZnO can be abstracted from the total spectrum for Emulsion E.
Conclusion:
Preliminary results from studies of a series of commercially formulated cosmetic emulsions suggest that a technique based upon a combination of acoustic spectroscopy and electro-acoustics is a powerful new tool to characterize the stability and performance of cosmetic emulsions without the need for dilution. Measurements are made in real time and can follow dynamic changes as the system is perturbed. Particle (droplet) size and zeta potential can be easily measured on both O/W and W/O systems; as long as the emulsion can be pumped it can be measured. Indeed, for the systems in the present study, it should be possible to measure them in-line. Based on the present work many avenues of investigation suggest themselves. Future studies will probe in more detail the ability of the technique to discriminate the presence of a particulate phase within the emulsion and then to follow this as a function of homogenization. It would be interesting to determine, for a given emulsion, the effect of HLB of emulsifier.
References:
1. J. Lyklema, “Fundamentals of Interface and Colloid Science”, Vol. 1, Academic Press (1993).
2. D. Fairhurst, Tire Technology International, 151-154 (1996).
3. N. G. Stanley-Wood and R.W.Line (Eds.), “Particle Size Analysis”, Royal Society of Chemistry (1992).
4. R. J. Hunter, “Zeta Potential in Colloid Science”, Academic Press (1981).
5. H.G. Barth (Ed.), “Modern Methods of Particle Size Analysis”, Wiley Interscience, (1984)
6. Th. Provder (Ed.), ACS Symposium Series 332 (1987), Series 472 (1991) and Series 693 (1996).
7. B. J. Marlow, D. Fairhurst and H. P. Pendse, Langmuir, 4, 611-626 (1983).
8. D. J. McClements, Colloids and Surfaces, 37, 33-72 (1991).
9. F. Alba, C.L Dobbs and R.G.Spark, First International Particle Technology Forum, Part 1, 36-46 (1994).
10. R.W. O’Brien, D. W. Cannon and W. N. Rowlands, J. Coll. and Int. Sci., 173, 406-418 (1995).
11. A. S. Dukhin and P. J. Goetz, Langmuir, 12, 4336-4344 (1996).
12. A. S. Dukhin and P. J. Goetz, Colloids and Surfaces, 144, 49-58 (1998).
13. T. H. Wines, A. S. Dukhin and P. Somasundaran, J. Coll and Int. Sci., 216, 303-308 (1999).
14. A. S. Dukhin et al, Colloids and Suraces A, 173, 127-159 (2000).
15. A. S. Dukhin and P.J. Goetz, U.S. Patent 6109098 (2000).
Table 1
ProductEmulsionEmulsionInternal PhaseViscosityNumber of
TypeStabilizerWt. FractionComponents
AO/WAnionic19%13,800cP9
BO/WNon-ionic32%4850cP13
CW/ONon-ionic74%134,000cP10
DO/WNon-ionic16%4600cP14
EO/WNon-ionic27%6,400cP18
Table 2
ProductMeasured Zeta Potential (mV)
A-20.0
B+4.5 (initial)
+3.0 (after adding SPAN20)
Figure 1.
Effect of shear on the attenuation spectra of an O/W anionic emulsion (A).
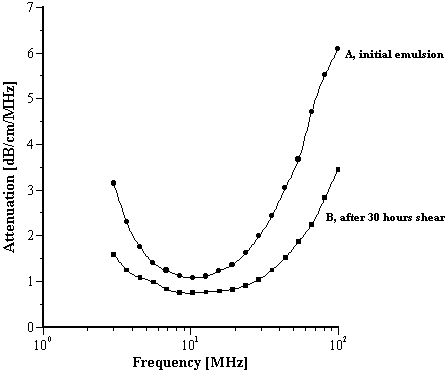
Figure 2.
Change of attenuation, at 4 MHz and 100 MHz, as a function of time of shear of the anionic emulsion A.
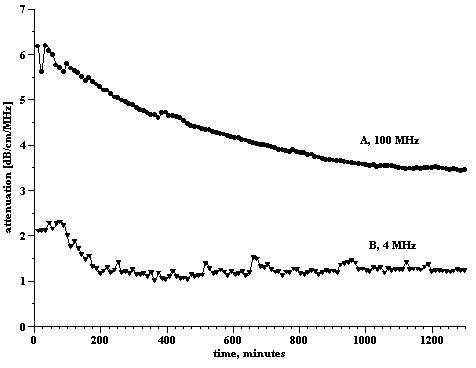
Figure 3.
Effect of shear on the droplet size distribution of the O/W anionic emulsion A.
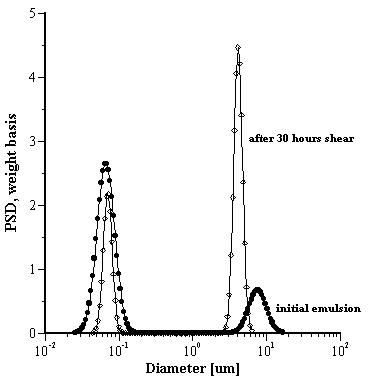
Figure 4. Effect of dilution on the attenuation spectra of a stable O/W emulsion (B).
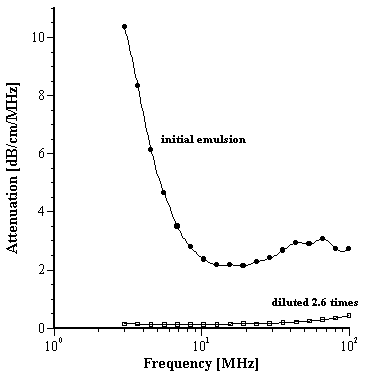
Figure 5.
Effect of addition of surfactant SPAN 20 on the diluted, de-stabilized O/W emulsion B.
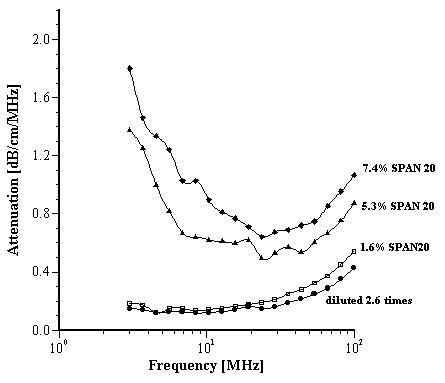
Figure 6.
“Stabilization” of an unstable (separated) W/O emulsion C after addition of surfactant
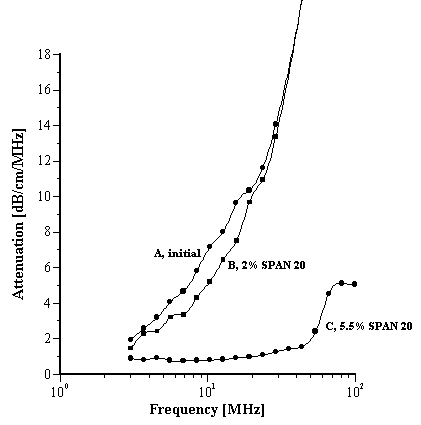
Figure 7.
Comparison of O/W Moisturiser Formulations.